Breadcrumb
- Home
- Research
Overview
The long-term goal of the Glykys Laboratory is to identify novel strategies to treat seizures and cytotoxic edema, especially in neonates.
To reach this goal, we are addressing the following themes:
- The workings of the brain's inhibitory system at the cellular and network level.
- Movement of water and ions into and out of neurons.
- Neuronal injury pathways after hypoxia, excitotoxic injury, and seizures.
Research areas of the lab include:
- Changes in neuronal chloride, calcium, and cellular volume during pathological conditions.
- Neonatal seizures and epilepsy.
- GABAA receptor physiology.
We approach these research areas with electrophysiological and two-photon imaging techniques in the neocortex. We complement these approaches with immunohistochemistry.
Learn more by checking these recent publications:
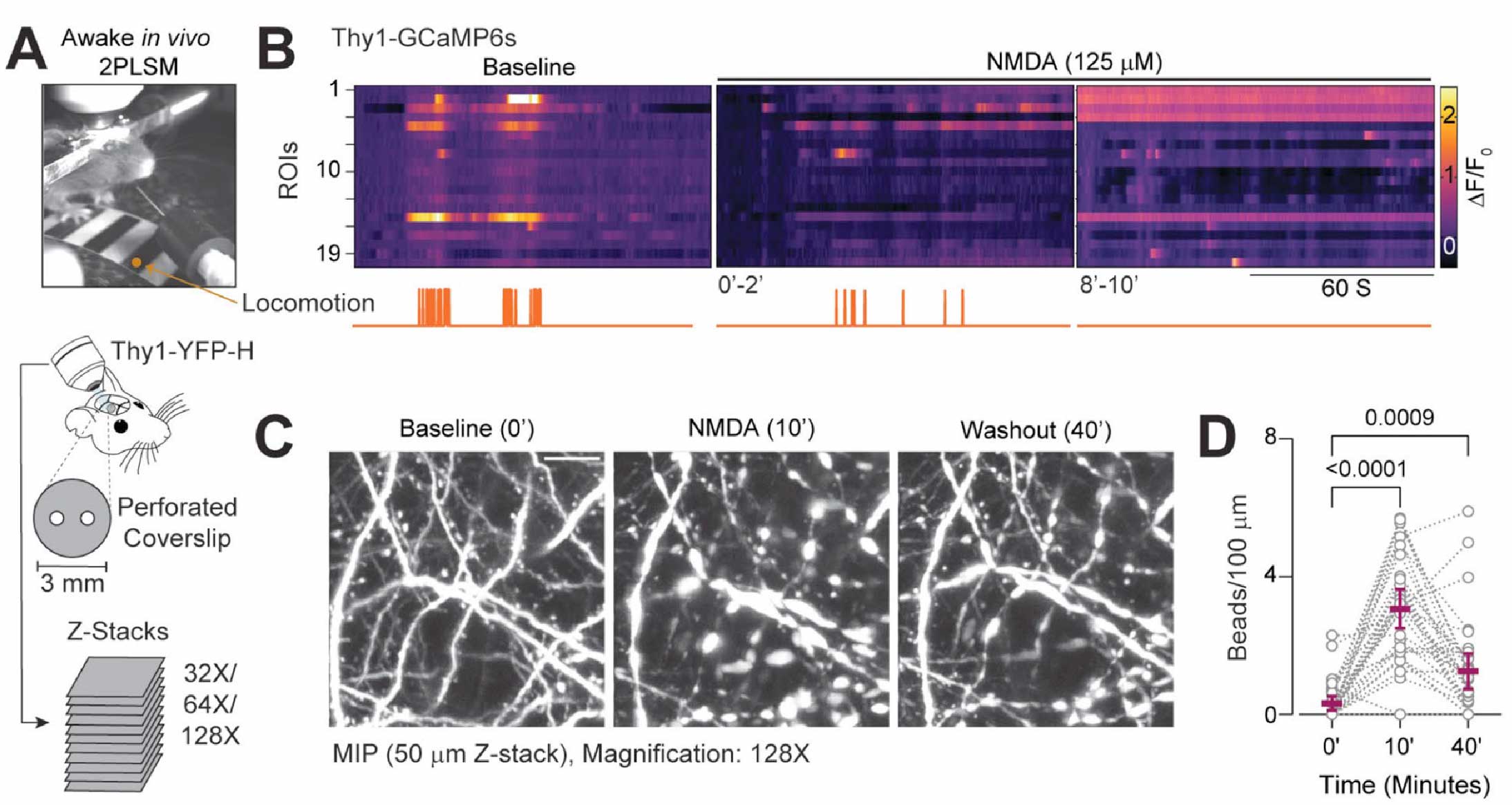
Suryavanshi P, Tadinada SM, Baule S, Jhaveri-Cruz N, Abel T, Glykys J. Dendritic beading during early brain development impairs signal transmission and synaptic plasticity. Acta Neuropathol Commun. 2025 Oct 6;13(1):212. https://link.springer.com/article/10.1186/s40478-025-02123-8
| 
|
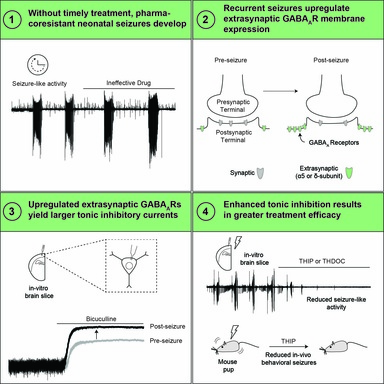
Liddiard GT, Buchanan GF, Schultz ML, Glykys J. Recurrent neonatal seizures increase tonic inhibition and respond to enhancers of δ-containing GABAA receptors. JCI Insight. 2025 Sep 16;10(21):e196152. https://insight.jci.org/articles/view/196152
| 
|
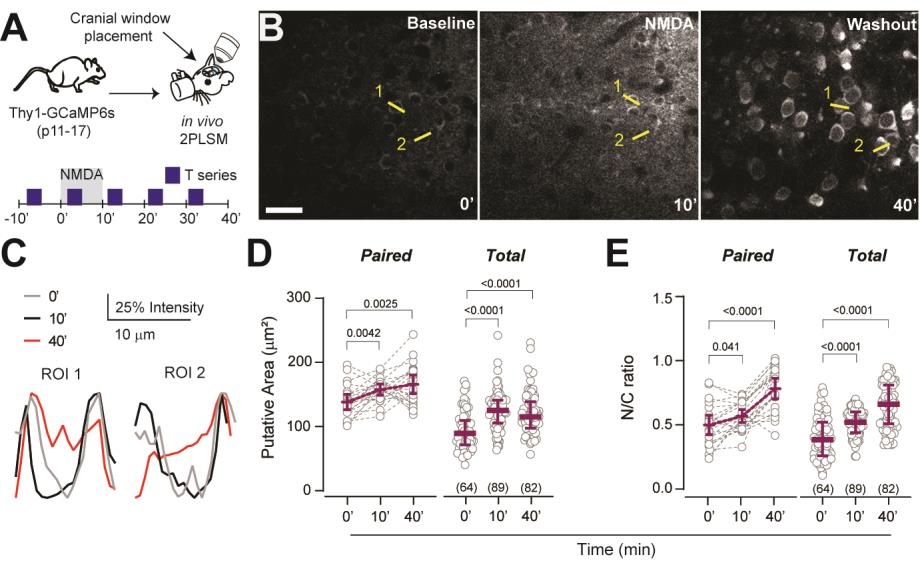
Suryavanshi P, Langton R, Fairhead K, Glykys J. Brief and diverse excitotoxic insults increase the neuronal nuclear membrane permeability in the neonatal brain, resulting in neuronal dysfunction and cell death. J Neurosci. 2024 Aug 30:e0350242024. doi: 10.1523/JNEUROSCI.0350-24.2024. [Cover of J. Neuroscience journal Oct 30]
| 
|
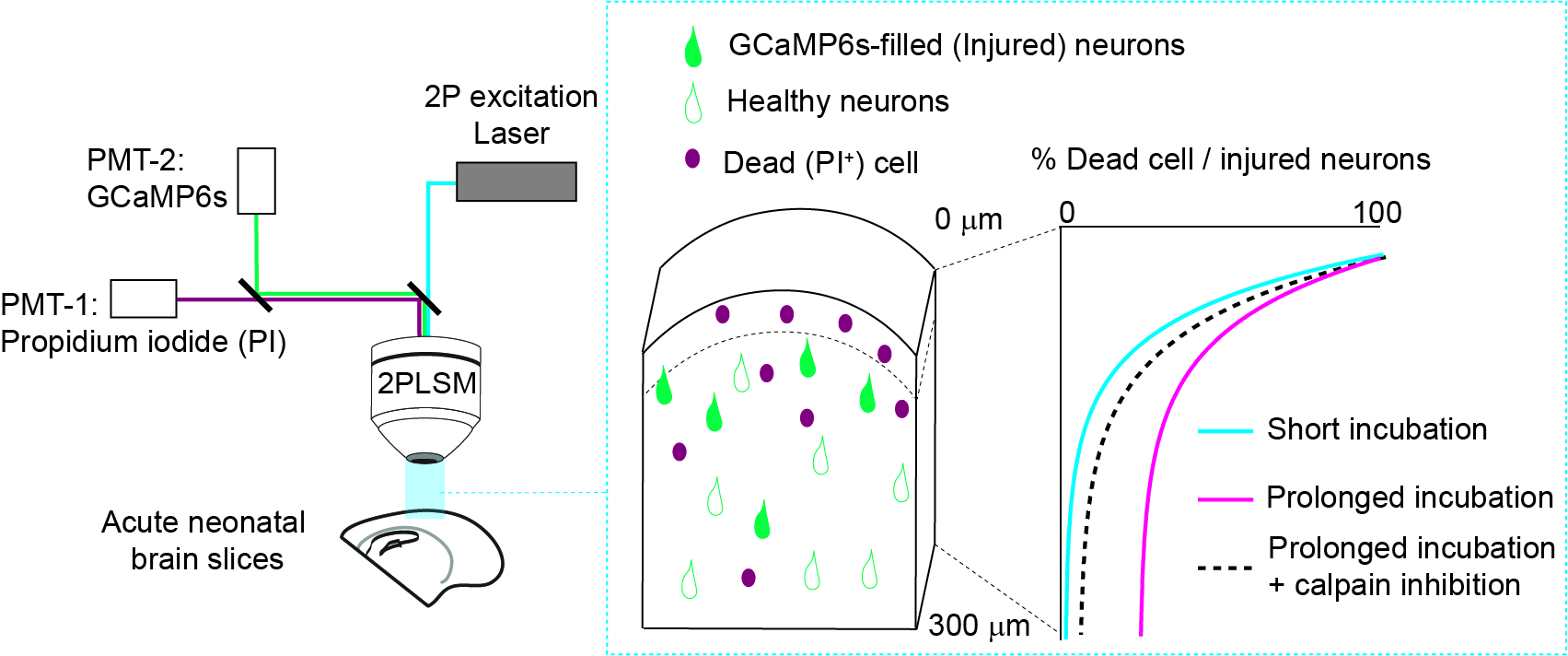
Suryavanshi P, Baule S, Glykys J. Trauma in neonatal acute brain slices alters calcium and network dynamics and causes calpain-mediated cell death. eNeuro 17 June 2024, ENEURO.0007-24.2024; https://doi.org/10.1523/ENEURO.0007-24.2024
| 
|
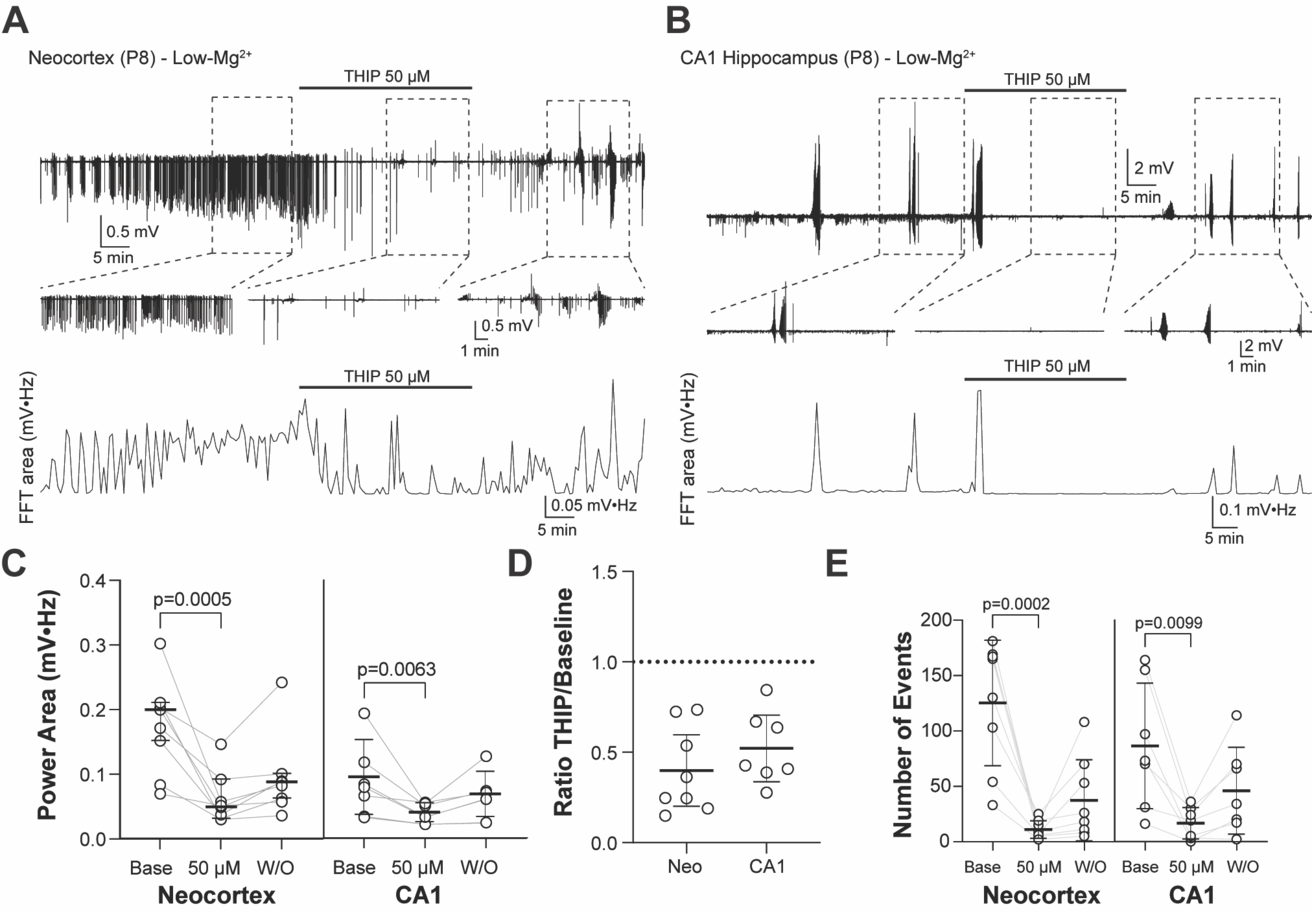
Liddiard GT, Suryahvanshi PS, Glykys J. Enhancing GABAergic tonic inhibition reduces seizure-like activity in the neonatal mouse hippocampus and neocortex. Journal of Neuroscience 4 January 2024, e1342232023; DOI: https://doi.org/10.1523/JNEUROSCI.1342-23.2023
| 
|
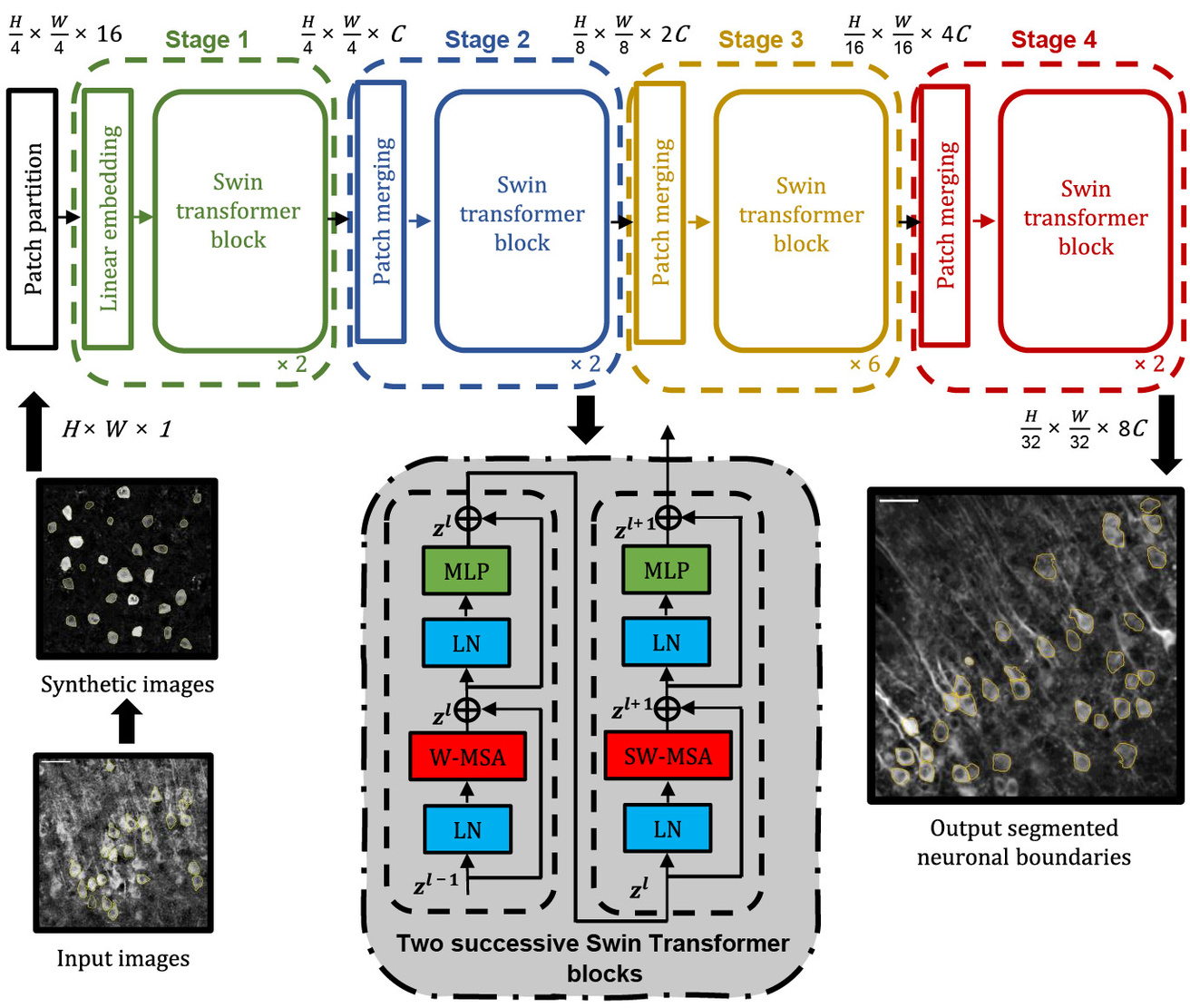
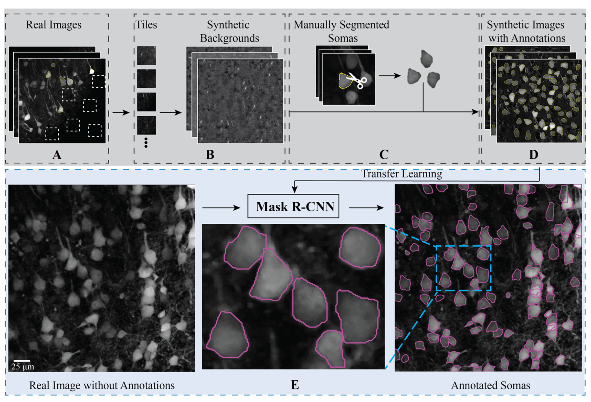
Islam MS, Suryavanshi P, Baule SM, Glykys J*, Baek S*. A Deep Learning Approach for Neuronal Cell Body Segmentation in Neurons Expressing GCaMP using a Swin Transformer. eNeuro. 2023 Sep 13; ENEURO.0148-23.2023. PMID: 37704367. [*, Corresponding authors]
| 
|
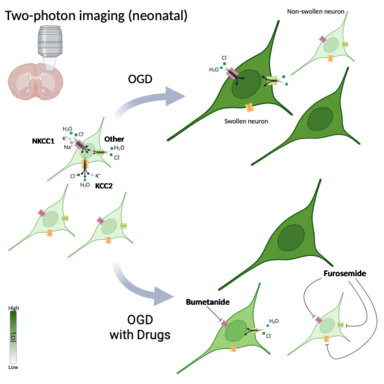
Takezawa Y, Langton R, Baule SM, Zimmerman MB, Baek J. Glykys, J. Role of NKCC1 and KCC2 during hypoxia-induced neuronal swelling in the neonatal neocortex. Neurobiol. Dis. 2023. Mar;178:106013. PMID: 36706928
| 
|
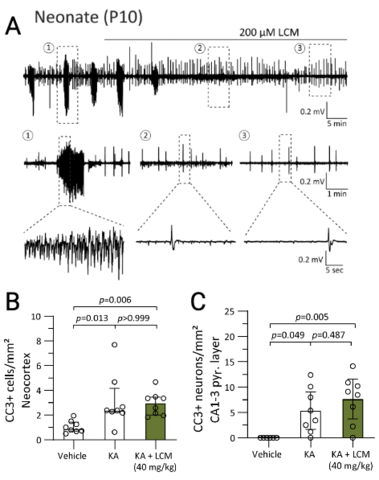
Langton R, Sharma S, Tiarks GC, Bassuk AG, Glykys, J. Lacosamide decreases neonatal seizures without increasing apoptosis. Epilepsia. 2022. Dec;63(12):3051-3065. PMID: 36168798
| 
|
Tong L, Langton R, Glykys J, Baek S. ANMAF: An automated neuronal morphology analysis framework using convolutional neural networks. Scientific Reports. 2021 April 14;11(1):8179. PMID: 33854113.
| 
|